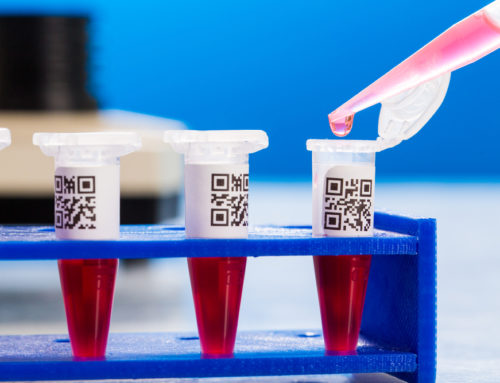
Pharmaceutical companies must be compliant with new changes to the Drug Supply Chain Security Act. This law, known as DSCSA, requires drug companies to serialize all packaging in case of recalls or investigations.
Serialization of this kind is also required by the European Union for pharmaceuticals being sold overseas. These required labels are essential for what is called “track and trace”, which is the process of finding current and past locations (and other information) of a unique item.
With serial numbers and barcodes, this process is made possible. Serialization offers a quick and easy security feature. It gives each individual item is own serial number, which eliminates the chances of mix ups.
At ABLT, we produce custom serialized labels for a number of companies, including many in the medical field. Below is an example of a label that can be used to give information about what the product is, where it was produced, and when it was produced.

Is your label design in need of digital serialization? Give us a call at 1-800-321-3653 or send us an email.